Mindray iMEC-12
Device Name:
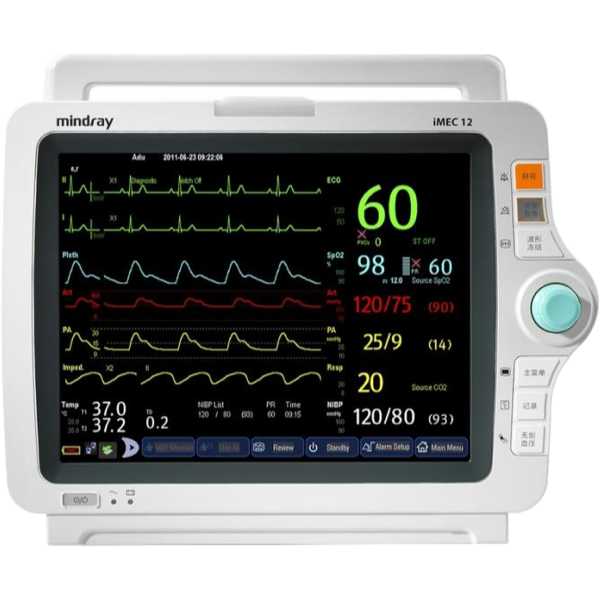
iMEC-12
Manufacturer:
Brand: SOMA Technology Inc., 166 Highland Park Drive, Bloomfield, CT 06002, UNITED STATES.
OBL: Mindray North America, 800 Macarther Blvd, Mahwah, NJ 07430, UNITED STATES.
Measuring functions:
Blood Pressure, SpO2, PR, ECG, Temperature and Respiration
Primary Client Use:
Intended for patient monitoring
Measurement Site:
BP: Arm and Intra-Arterial; SpO2: Finger
Measurement Occurrence:
BP: Continuous and intermittent measurements; SpO2: Continuous measurement
Availability:
Available Currently
Device Specifications:
Description:
The Mindray iMEC-12 is a patient monitor. The accuracy of its blood pressure measurement technology has yet to be proven to MDR requirements and information regarding the accuracy of its oxygen saturation measurement technology does not appear to be available. Both upper arm and intra-arterial measurements can be taken and oxygen saturation measurements are taken from the finger. It is intended for bedside patient monitoring.
Assessment:
While the technology used in the Mindray iMEC-12, to measure blood pressure, has been declared as being equivalent to that used in another device, not only has no evidence has been published to show that the devices have been compared according to a protocol compliant with (EU) 2017/745 and MEDDEV 2.7/1 rev 4, but the results of the assessment on which the declaration has been based have not been published either and cannot be verified. However, there are validation studies proving clinical validation of the same measurement technology, according to other equivalence claims. There appears to be no peer-reviewed clinical validation information available on the technology used in the Mindray iMEC-12 to measure peripheral oxygen saturation.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | None | Although equivalence to another device is claimed, this has not been tested to MDR requirements. |
| BP | BIHS (UK and IRL) | Hospital use | Manufacturer declaration of equivalence to the Mindray BeneView T5 without published evidence |
| BP | ESH (Europe) | Professional use | The BIHS recommendation, despite the absence, at the time, of the scientific evidence required by the same authors' criteria. |
Device Family:
Datascope iPM 12B, Mindray iMEC-12B, Mindray Beneview T5B, Mindray BeneVision N12B
Legend: B BIHS Derivative
Legend: B BIHS Derivative
