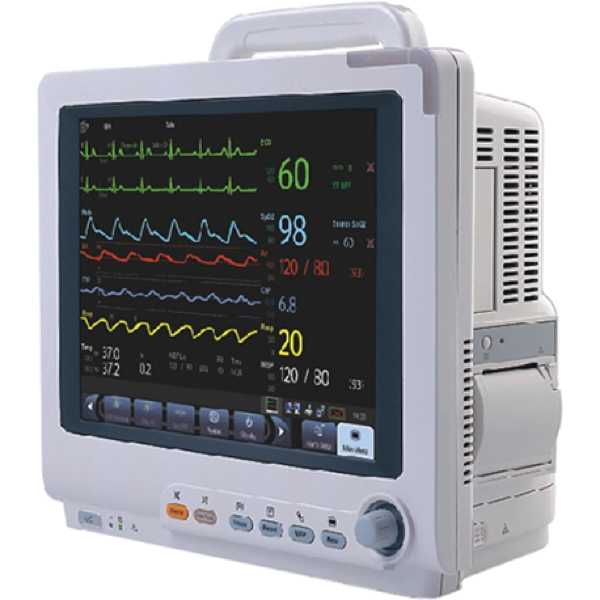
Mindray Beneview T5
Device Name:
Beneview T5
Manufacturer:
Brand: SOMA Technology Inc., 166 Highland Park Drive, Bloomfield, CT 06002, UNITED STATES.
OBL: Mindray North America, 800 Macarther Blvd, Mahwah, NJ 07430, UNITED STATES.
OEM: SOMA Technology Inc., 166 Highland Park Drive, Bloomfield, CT 06002, UNITED STATES.
Measuring functions:
Blood Pressure, SpO2, PR, ECG, Temperature, Respiration and Arrhythmias
Primary Client Use:
Intended for patient monitoring
Measurement Site:
BP: Arm and Intra-Arterial; SpO2: Finger
Measurement Occurrence:
BP: Continuous and intermittent measurements; SpO2: Continuous measurement
Availability:
Available Currently
Device Specifications:
Description:
The Mindray Beneview T5 is a patient monitor. The accuracy of its blood pressure measurement technology has yet to be proven to MDR requirements and information regarding the accuracy of its oxygen saturation measurement technology does not appear to be available. Both upper arm and intra-arterial measurements can be taken and oxygen saturation measurements are taken from the finger. It is intended for bedside patient monitoring.
Assessment:
While the technology used in the Mindray Beneview T5, to measure blood pressure, has been assessed, the results of the study have not been published and cannot be verified. There appears to be no peer-reviewed clinical validation information available on the technology used in the Mindray Beneview T5 to measure peripheral oxygen saturation.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | None | While a clinical validation is claimed, it cannot be checked properly. |
| BP | BIHS (UK and IRL) | Hospital use | Unpublished internal data |
| BP | ESH (Europe) | Professional use | The BIHS recommendation, despite the absence, at the time, of the scientific evidence required by the same authors' criteria. |
Device Family:
Datascope iPM 12B, Mindray iMEC-12B, Mindray Beneview T5B, Mindray BeneVision N12B
Legend: B BIHS Derivative
Legend: B BIHS Derivative
