Terumo ES-P2020ZZ
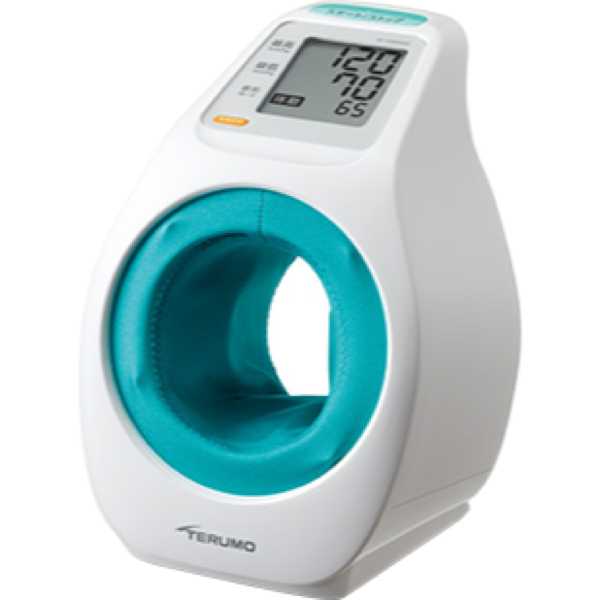
Device Model:
ES-P2020ZZ
Manufacturer:
Terumo Corporation, 2-44-1 Hatagaya, Shibuya-ku, Tokyo 151-0072, JAPAN.
Measuring functions:
Blood Pressure and PR
Primary Client Use:
Intended for use as a public facility
Measurement Site:
Upper Arm
Measurement Occurrence:
Single measurements only
Availability:
Available Currently
Availability according to Countries or Regions:
Japan
Device Manual:
Description:
The Terumo ES-P2020ZZ is a waiting-room blood pressure monitor. The accuracy of its blood pressure measurement technology has yet to be proven to MDR requirements. Blood pressure measurements are taken from the upper arm. It is intended for use as a public facility.
Assessment:
While the technology used in the Terumo ES-P2020ZZ, to measure blood pressure, has been assessed, the results of the study have not been published and cannot be verified.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | None | While a clinical validation is claimed, it cannot be checked properly. |
| BP | Japanese Society of Hypertension | Self-measurement (Previous: 2017-2019) | Possibly the intended device, listed as Terumo ES-P2020, to which an internal validation of the Terumo ES-P2000 is applied without explanation |
Device Family:
Validation Publications for Equivalent Devices:
Note: This is a provisional list, as equivalence according to EU Regulation 2017/745 (e.g. MEDDEV 2.7/1 rev 4) is not proven. Accordingly, these publications are not used in the assessment of star-ratings.
Terumo ES-P2000
血圧計に関するワーキンググループ, 日本高血圧学会学術委員会 [Working Group on Sphygmomanometers, Academic Committee on Hypertension in Japan] [Test reports on the Terumo R800 and P2000] [Internet] Tokyo: JSH; 2010. Available from: www.jpnsh.jp.
JIS T 1115:2005 General population
JIS T 1115:2005 General population
There is insufficient information provided in either of two reports to enable scientific assessment.
