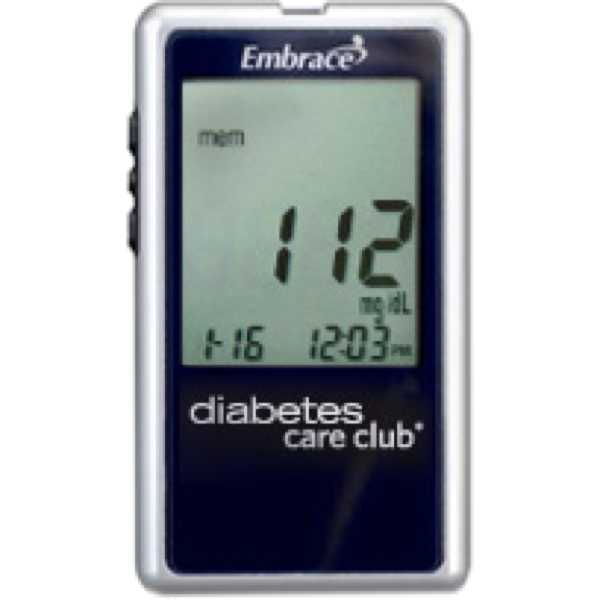

OBL: Omnis, 505 Airpark Center Drive, Nashville, TN 37217, UNITED STATES.
OEM: Apex Biotechnology Corp., 7 Li-Hsin Road V, Hsinchu Science Park, Hsinchu 30078, TAIWAN ROC.
| Accuracy Assessment | Recommendation | Basis | |
| BG | Medaval | Not recommended | This device has failed a clinical validation. |
| BG | Institute of Health Economics (CA) | General use | No evidence provided |
Brazg RL, Klaff LJ, Parkin CG. Performance variability of seven commonly used self-monitoring of blood glucose systems: clinical considerations for patients and providers. J Diabetes Sci Technol. 2013 Jan 1;7(1):144-52. doi: 10.1177/193229681300700117. PMID: 23439170. Available from: PMC3692226.
15197:2003 - Fail General population
Klonoff DC, Parkes JL, Kovatchev BP, Kerr D, Bevier WC, Brazg RL, Christiansen M, Bailey TS, Nichols JH, Kohn MA. Investigation of the Accuracy of 18 Marketed Blood Glucose Monitors. Diabetes Care. 2018 Aug;41(8):1681-8. Epub: 2018 Jun 13. doi: 10.2337/dc17-1960. PMID: 29898901. Available from: www.diabetestechnology.org.
DTS:2016 - Fail General population
