OBL: K-jump Health Co. Ltd., 56 Wu Kung 5th Rd., New Taipei Industrial Park, New Taipei City 24890, TAIWAN ROC.
OEM: Spengler S.a.s., 190 rue Paul Langevin, ZAC La Robole, 13856 Aix-en-Provence, FRANCE.
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | None | Although equivalence to another device is claimed, this has not been tested to MDR requirements. |
Legend: B BIHS Derivative, D DET Equivalence
Note: This is a provisional list, as equivalence according to EU Regulation 2017/745 (e.g. MEDDEV 2.7/1 rev 4) is not proven. Accordingly, these publications are not used in the assessment of star-ratings.
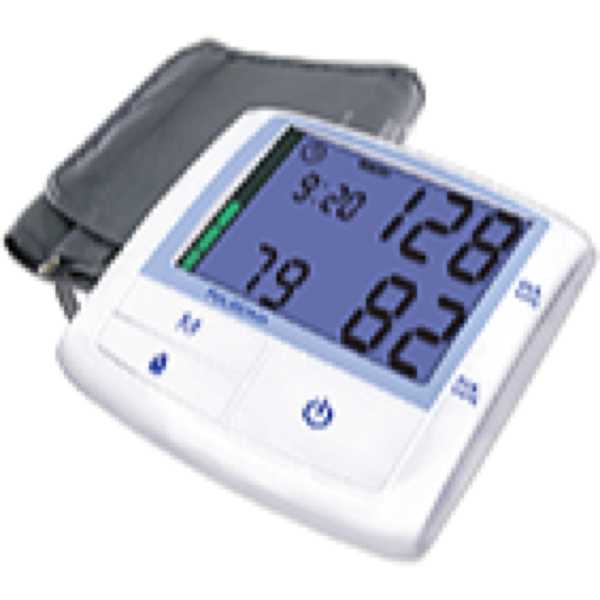
Spengler KP7500 D
Belghazi J, El Feghali RN, Moussalem T, Rejdych M, Asmar RG. Validation of four automatic devices for self-measurement of blood pressure according to the International Protocol of the European Society of Hypertension. Vasc Health Risk Manag. 2007;3(4):389-400. PMID: 17969368. Available from: PMC2291343.
ESH-IP:2002 - Pass General population
