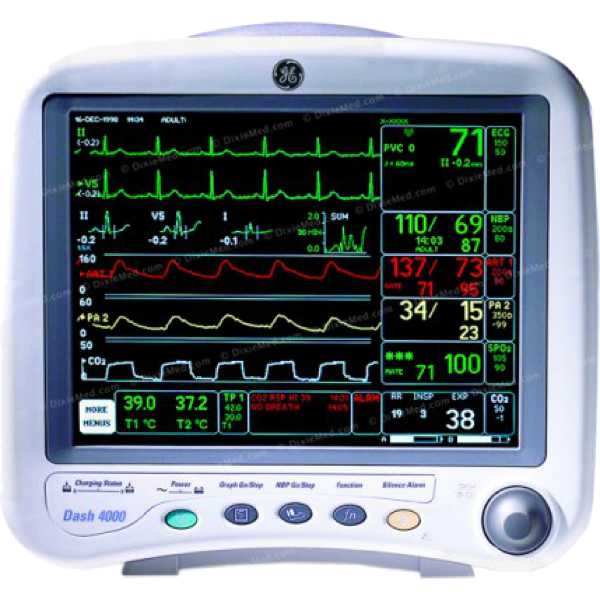
GE Dash 4000
Device Name:
Dash 4000
Manufacturer:
General Electric Company, 33-41 Farnsworth Street, Boston, MA 02210, UNITED STATES.
Measuring functions:
Blood Pressure, SpO2, PR, ECG, Temperature, Respiration, EtCO2, SvO2, Cardiac Output, ICG, BIS and Anaesthsia Gases
Primary Client Use:
Intended for patient monitoring
Measurement Site:
BP: Arm and Intra-Arterial; SpO2: Finger
Measurement Occurrence:
BP: Single, intermittent and continuous measurements; SpO2: Continuous measurement
Availability:
Available Currently
Device Manual:
Description:
The GE Dash 4000 is a patient monitor. Its blood pressure measurement technology has been proven to be accurate and information regarding the accuracy of its oxygen saturation measurement technology does not appear to be available. Both upper arm and intra-arterial measurements can be taken and oxygen saturation measurements are taken from the finger. It is intended for bedside patient monitoring.
Assessment:
The technology used in the GE Dash 4000, to measure blood pressure, has passed in a clinical validation study, in a general population, according to a recognised standard protocol, as published in a peer-reviewed publication. There appears to be no peer-reviewed clinical validation information available on the technology used in the GE Dash 4000 to measure peripheral oxygen saturation.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | Legacy approval | Older clinical validation; older protocol |
Validation Publications:
Hersh LT, Sesing JC, Luczyk WJ, Friedman BA, Zhou S, Batchelder PB. Validation of a conical cuff on the forearm for estimating radial artery blood pressure. Blood Press Monit. 2014 Feb;19(1):38-45. doi: 10.1097/MBP.0000000000000011. PMID: 24217368.
81060-2:2009 - Pass General population (Note: IA study (n=34))
