Philips IntelliVue MX-400

Device Name:
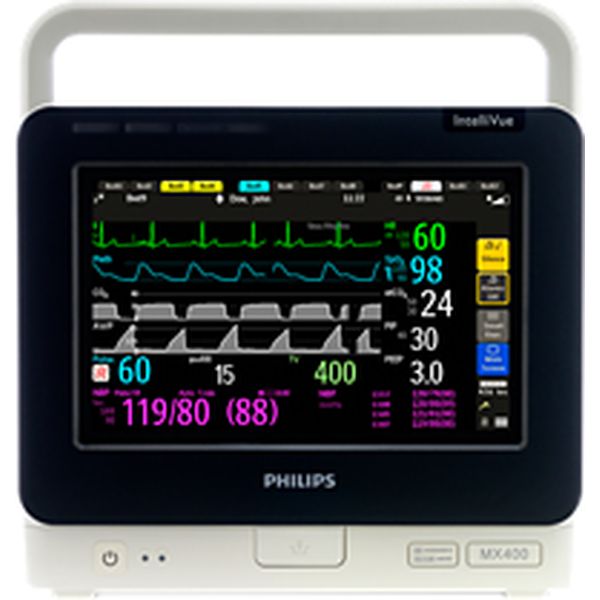
IntelliVue MX-400
Manufacturer:
Philips Electronics North America Corp., 3000 Minuteman Road, M/S 109, Andover, MA 01810, UNITED STATES.
Measuring functions:
Blood Pressure, SpO2, PR, ECG, Temperature, Respiration, CO2, SvO2, Spirometry, Airway flow, Arrhythmias, Cardiac Output, EEG, BIS, NMT and tcGas
Primary Client Use:
Intended for patient monitoring
Measurement Site:
BP: Arm and Intra-Arterial; SpO2: Finger
Measurement Occurrence:
BP: Single, intermittent and continuous measurements; SpO2: Continuous measurement
Availability:
Available Currently
Availability according to Countries or Regions:
Japan
Device Manual:
Device Specifications:
Description:
The Philips IntelliVue MX-400 is a patient monitor. Medaval has not found evidence proving the accuracy of its blood pressure measurement technology and information regarding the accuracy of its oxygen saturation measurement technology does not appear to be available. Both upper arm and intra-arterial measurements can be taken and oxygen saturation measurements are taken from the finger. It is intended for bedside patient monitoring.
Assessment:
There appears to be no peer-reviewed clinical validation information available on the technology used in the Philips IntelliVue MX-400 either to measure blood pressure or to measure peripheral oxygen saturation.
Recommendations:
| Accuracy Assessment | Recommendation | Basis | |
| BP | Medaval | Not recommended | This device has not been clinically validated. |
| BP | Japanese Society of Hypertension | Professional use (Previous: 2018) | No evidence provided |